

1 As of August 2017, the FreeStyle Libre system has been commercially available in Canada.
#Freestyle libre flash glucose monitoring system fda manual#
įreeStyle Libre Pro flash glucose monitoring system: operator’s manual Alameda (CA): Abbott Diabetes Care Inc. The consumer version of the FreeStyle Libre system intended for personal use by patients with diabetes received regulatory approval in Canada (June 2017) and the US (September 2017). Abbotts revolutionary device is called the FreeStyle. It’s called the FreeStyle Libre Flash Glucose. Ambulatory glucose profile: Flash glucose monitoring. FDA approval for a new Blood Glucose Monitoring (BGM) system has the diabetes community celebrating. Food and Drug Administration (FDA) has approved a device that will bring diabetes patients into the 21st century of at-home medical care. įreeStyle Libre Pro Alameda (CA): Abbott Diabetes Care 2016. MAUDE Adverse Event Report: ABBOTT DIABETES CARE INC FREESTYLE LIBRE 2 FLASH GLUCOSE MONITORING SYSTEM. This sleek, easy to read meter goes with you and delivers fast, accurate results from a tiny blood sample. It is designed to replace blood glucose testing for diabetes treatment decisions. 'Studies have shown that a majority of people test less than three times per day because of the pain and hassles associated with finger sticks. The FreeStyle Libre Flash Glucose Monitoring System is a continuous glucose monitoring (CGM) device indicated for the management of diabetes in persons age 18 and older. 2013 Apr 37(Suppl 1):S35–S39.Ībbott receives FDA approval for the FreeStyle Libre Pro™ system, a revolutionary diabetes sensing technology for healthcare professionals to use with their patients Abbott Park (IL): Abbott 2016. FreeStyle Lite Blood Glucose Monitoring System It’s small. The FDA has approved the FreeStyle Libre Flash Glucose Monitoring System, which eliminates the need for routine finger sticks to check blood glucose levels.

But it only transmits glucose levels to the reader when you scan it. This means the device is monitoring your sugar at all times.
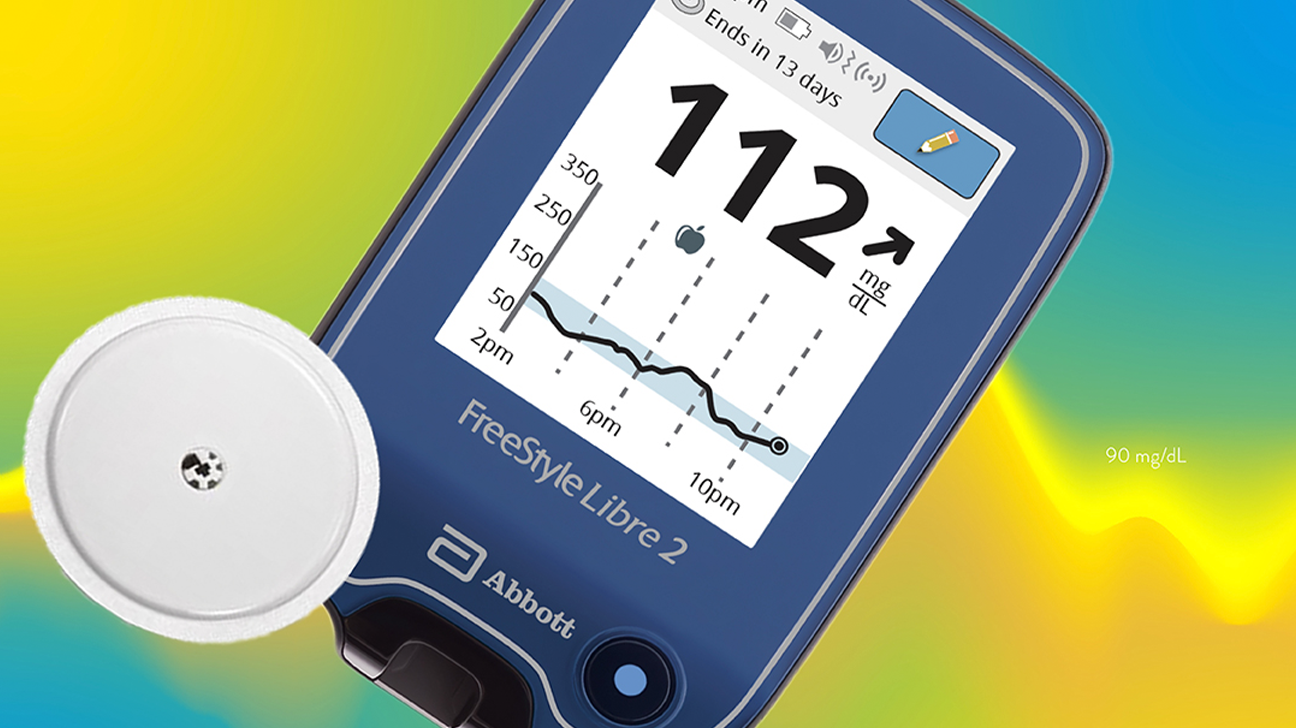

However, these systems are known as flash glucose monitors. Berard LD, Blumer I, Houlden R, Miller D, Woo V. FreeStyle Libre 2 takes a reading every minute, and FreeStyle Libre 14 Day tracks your glucose every 15 minutes. You are permitted to reproduce this document for non-commercial purposes, provided it is not modified when reproduced and appropriate credit is given to CADTH.Ĭanadian Diabetes Association Clinical Practice Guidelines Expert Committee. The available evidence suggests that the performance and accuracy of the FreeStyle Libre Pro system is comparable to conventional methods of glucose monitoring, that it may reduce periods of hypoglycemia, or low blood sugar, and that it has high patient satisfaction.Īlthough the FreeStyle Libre Pro system is approved for use in Canada, it is not yet commercially available here.Ĭanadian pricing is not available, but cost-effectiveness analyses conducted in the UK and other European countries have concluded that it is cost-effective compared with the self-monitoring of blood glucose.Ī consumer version of the FreeStyle Libre Pro system intended for personal use by patients with diabetes is currently awaiting regulatory approval in the US.Ĭopyright © CADTH 2017. Food & Drug Administration, 2017 (Available at:). This site is not affiliated with or endorsed by the FDA.The FreeStyle Libre Pro is a glucose monitoring system intended to replace finger-stick tests it uses a sensor implanted in the arm that a health care provider scans with a specialized reader for a record of glucose levels, trends, and patterns in people with diabetes. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. Process Change - Manufacturer/sterilizer/packager/supplierĪbbott Diabetes Care Inc. The sensor container and sensor kit are components of the freestyle libre pro flash continuous glucose monitoring (cgm) device DeviceįreeStyle Libre Pro Flash Glucose Monitoring System Approval to add an alternate manufacturing site for sensor container assembly and finished sensor kit packing.


 0 kommentar(er)
0 kommentar(er)
